Abstract
Introduction: Ponatinib is a third-generation, pan-inhibitory tyrosine kinase inhibitor (TKI) designed to potently inhibit BCR-ABL1 with or without any single resistance mutation, including T315I. Patients (pts) with resistant disease or with T315I BCR-ABL1 mutations respond inadequately to earlier-generation BCR-ABL1 TKIs, leading to poor survival outcomes. OPTIC (Optimizing Ponatinib Treatment in CP-CML, NCT02467270; ongoing) is a phase 2 trial evaluating the safety and efficacy of ponatinib in pts with CP-CML whose disease is resistant to 2 or more TKIs or who have a T315I mutation. Here, we present an in-depth post hoc analysis of OPTIC trial pt responses by baseline disease burden and mutation status.
Methods: Pts with CP-CML resistant to ≥2 TKIs or with the BCR-ABL1 T315I mutation were randomized to ponatinib starting doses of 45 mg (cohort A; 45 mg → 15 mg), 30 mg (B; 30 mg → 15 mg), and 15 mg (C) once daily. Doses were reduced to 15 mg after achievement of ≤1% BCR-ABL1 IS in cohorts A and B. Pts could re-escalate to their original starting dose for loss of response. The primary endpoint is ≤1% BCR-ABL1 IS at 12 months; secondary endpoints include cytogenetic and molecular responses and safety outcomes. In this analysis, outcomes are analyzed by baseline T315I mutation status and baseline disease burden in the intent-to-treat (ITT) population. BCR-ABL1 mutations were assessed by Sanger sequencing at a central laboratory (MolecularMD, Portland, OR, USA).
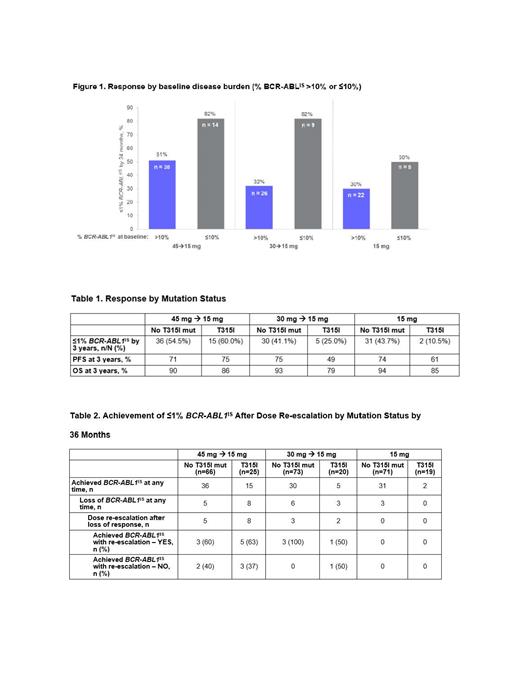
Results: 283 pts were randomized (A/B/C: n=94/95/94). At baseline, 84.1% of pts had a high (>10% BCR-ABL1 IS) disease burden; 23.8% had T315I mutation, 17.0% had a mutation other than T315I, and 57.8% had no mutation. The 45 mg →15 mg cohort showed the highest ≤1% BCR-ABL1 IS response rates by 36 months (Figure 1).
Subanalysis of pts across the 3 dosing arms showed that pts with T315I mutations had the highest ≤1% BCR-ABL1 IS response rates (60%) by 3 years with the 45 mg → 15 mg dose compared with the other cohorts, with a trend toward higher progression-free survival (PFS) in the 45 mg →15 mg arm (Table 1). Across all 3 cohorts, 97 pts without T315I mutations (ie, no mutation or with mutations other than T315I) achieved ≤1% BCR-ABL1 IS. Median duration of response (mDoR) for pts with a T315I mutation at baseline was 27 months for pts (n=15) in the 45 mg → 15 mg cohort and 12 mo for pts (n=5) in the 30 mg → 15 mg cohort. For pts without T315I mutations, the mDoR was not reached. Across all 3 cohorts, 79% of pts who achieved ≤1% BCR-ABL1IS maintained this response during the study. A total of 25 patients lost responses (Table 2). Of those who lost response, 11 had T315I, 10/11 dose re-escalated; of those who re-escalated, 6/10 regained ≤1% BCR-ABL1IS after dose re-escalation (Table 2). The most common nonhematologic treatment-emergent adverse events (TEAEs) in the ITT population for all cohorts combined were arterial hypertension (28%), headache (18%), and lipase increased (17%). The most common hematologic TEAEs were thrombocytopenia (40%), neutropenia (26%), and anemia (19%). Overall, 6.0% of pts experienced a treatment-emergent arterial occlusive event (TE-AOE); 4.6% experienced a Grade ≥3 TE-AOE.
Conclusions: Previous analyses established these largely resistant pts achieved high response rates when treated with ponatinib. Consistent with this, the OPTIC post hoc analysis showed clinical benefit across all 3 dosing regimens regardless of T315I mutation status at baseline; the 45 mg →15 mg cohort showed the highest response rates regardless of baseline disease burden (as assessed by BCR-ABL1 IS levels). Regardless of T315I mutation status, most patients were able to maintain their response after dose reduction to 15 mg/day upon achieving BCR-ABL1IS ≤1%. Compared with patients without a T315I mutation, patients with a T315I mutation at baseline were more likely to lose their response upon dose reduction; however, 60% of responses were regained with dose re-escalation. For pts with T315I mutations, PFS was greater with 45 mg → 15 mg dosing compared with the other arms; for pts without T315I mutations, all 3 doses showed robust PFS and OS outcomes. The data presented further support the benefit of using ponatinib post-2nd generation TKI for pts with resistant disease regardless of baseline T315I mutation status.
Deininger: Incyte: Consultancy, Honoraria, Research Funding; Fusion Pharma, Medscape, DisperSol: Consultancy; Blueprint Medicines Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Part of a Study Management Committee, Research Funding; SPARC, DisperSol, Leukemia & Lymphoma Society: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Part of a Study Management Committee, Research Funding; Sangamo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding. Apperley: Incyte, Pfizer: Honoraria, Research Funding, Speakers Bureau; Bristol Myers Squibb, Novartis: Honoraria, Speakers Bureau. Chuah: Steward Cross: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Pfizer: Other: Travel, Research Funding; Novartis, Korea Otsuka Pharmaceutical: Honoraria. Hochhaus: Bristol-Myers Squibb: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Incyte: Research Funding. De Lavallade: Pfizer, Novartis.: Honoraria; Bristol Myers Squibb, Incyte: Honoraria, Research Funding. Lipton: Bristol Myers Squibb, Ariad, Pfizer, Novartis: Consultancy, Research Funding. Lomaia: Novartis: Honoraria; Pfizer: Honoraria; BMS: Honoraria; Pharmstandard: Honoraria. McCloskey: Takeda: Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau; BMS: Honoraria, Speakers Bureau; COTA: Other: Equity Ownership; Incyte: Speakers Bureau; Amgen: Speakers Bureau; Novartis: Consultancy; Pfizer: Consultancy. Mauro: Bristol Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy; Novartis: Consultancy, Research Funding; Sun Pharma / SPARC: Research Funding; Pfizer: Consultancy. Moiraghi: Novartis, Pfizer, Takeda: Speakers Bureau. Pavlovsky: Novartis, BMS, Pfizer, Takeda: Speakers Bureau. Rosti: Pfizer: Research Funding, Speakers Bureau; Bristol Myers Squibb, Incyte, Novartis: Speakers Bureau. Rousselot: Incyte, Pfizer: Consultancy, Research Funding. Undurraga: AbbVie, Janssen, Novartis, Pfizer, Roche: Other: Advisory Board; Janssen, Novartis, Pfizer: Speakers Bureau. Lu: Takeda: Current Employment. Vorog: Takeda: Current Employment. Cortes: Takeda: Consultancy, Research Funding; Sun Pharma: Consultancy, Research Funding; Bristol Myers Squibb, Daiichi Sankyo, Jazz Pharmaceuticals, Astellas, Novartis, Pfizer, Takeda, BioPath Holdings, Incyte: Consultancy, Research Funding; Bio-Path Holdings, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal